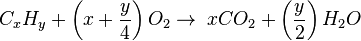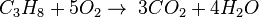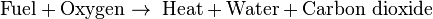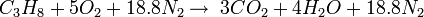Combustion
From Wikipedia, the free encyclopedia
Combustion or burning is a complex sequence of exothermic chemical reactions between a fuel and an oxidant accompanied by the production of heat or both heat and light in the form of either a glow or flames.
In a complete combustion reaction, a compound reacts with an oxidizing element, such as oxygen or fluorine, and the products are compounds of each element in the fuel with the oxidizing element. For example:
- CH4 + 2O2 → CO2 + 2H2O + heat
- CH2S + 6F2 → CF4 + 2HF + SF6 + heat
A simpler example can be seen in the combustion of hydrogen and oxygen, which is a commonly used reaction in rocket engines:
- 2H2 + O2 → 2H2O + heat
The result is simply water vapor.
In the large majority of the real world uses of combustion, the oxygen (O2) oxidant is obtained from the ambient air and the resultant flue gas from the combustion will contain nitrogen:
- CH4 + 2O2 + 7.52N2 → CO2 + 2H2O + 7.52N2 + heat
As can be seen, when air is the source of the oxygen, nitrogen is by far the largest part of the resultant flue gas.
In reality, combustion processes are never perfect or complete. In flue gases from combustion of carbon (as in coal combustion) or carbon compounds (as in combustion of hydrocarbons, wood etc.) both unburned carbon (as soot) and carbon compounds (CO and others) will be present. Also, when air is the oxidant, some nitrogen will be oxidized to various, mostly harmful, nitrogen oxides (NOx).
Contents
|
[edit] Types
[edit] Rapid
Rapid combustion is a form of combustion in which large amounts of heat and light energy are released, which often results in a fire. This is used in a form of machinery such as internal combustion engines and in thermobaric weapons.
Combustion is double replacement reaction. On the other hand a chemical reaction is single replacement reaction.
[edit] Slow
Slow combustion is a form of combustion which takes place at low temperatures. Respiration is an example of slow combustion.
[edit] Complete
In complete combustion, the reactant will burn in oxygen, producing a limited number of products. When a hydrocarbon burns in oxygen, the reaction will only yield carbon dioxide and water. When a hydrocarbon or any fuel burns in air, the combustion products will also include nitrogen. When elements such as carbon, nitrogen, sulfur, and iron are burned, they will yield the most common oxides. Carbon will yield carbon dioxide. Nitrogen will yield nitrogen dioxide. Sulfur will yield sulfur dioxide. Iron will yield iron(III) oxide. It should be noted that complete combustion is almost impossible to achieve. In reality, as actual combustion reactions come to equilibrium, a wide variety of major and minor species will be present. For example, the combustion of methane in air will yield, in addition to the major products of carbon dioxide and water, the minor products which include carbon monoxide, hydroxyl, nitrogen oxides, monatomic hydrogen, and monatomic oxygen.
[edit] Turbulent
Turbulent combustion is a combustion characterized by turbulent flows. It is the most used for industrial application (e.g. gas turbines, diesel engines, etc.) because the turbulence helps the mixing process between the fuel and oxidizer.
[edit] Incomplete
Incomplete combustion occurs when there isn't enough oxygen to allow the fuel (usually a hydrocarbon) to react completely with the oxygen to produce carbon dioxide and water, also when the combustion is quenched by a heat sink such as a solid surface or flame trap. When a hydrocarbon burns in air, the reaction will yield carbon dioxide, water, carbon monoxide, pure carbon (soot or ash) and various other compounds such as nitrogen oxides. Incomplete combustion is much more common and will produce large amounts of byproducts, and in the case of burning fuel in automobiles, these byproducts can be quite unhealthy and damaging to the environment.
Quality of combustion can be improved by design of combustion devices, such as burners and internal combustion engines. Further improvements are achievable by catalytic after-burning devices (such as catalytic converters). Such devices are required by environmental legislation for cars in most countries, and may be necessary in large combustion devices, such as thermal power plants, to reach legal emission standards.
[edit] Smoldering
Smouldering combustion is a flameless form of combustion, deriving its heat from heterogeneous reactions occurring on the surface of a solid fuel when heated in an oxidizing environment. The fundamental difference between smouldering and flaming combustion is that in smouldering, the oxidation of the reactant species occurs on the surface of the solid rather than in the gas phase. The characteristic temperature and heat released during smoldering are low compared to those in the flaming combustion of a solid. Typical values in smouldering are around 600 °C for the peak temperature and 5 kJ/g-O2 for the heat released; typical values during flaming are around 1500 °C and 13 kJ/g-O2 respectively. These characteristics cause smoulder to propagate at low velocities, typically around 0.1 mm/s, which is about two orders of magnitude lower than the velocity of flame spread over a solid. In spite of its weak combustion characteristics, smouldering is a significant fire hazard.
[edit] Combustion with other oxidants
Oxygen can be assumed as the oxidant when talking about combustion, but other oxidants exist. Nitrous oxide is used in rockets and in motorsport; it produces oxygen at over 1300 C. Fluorine, another oxidizing element, can produce a combustion reaction, to produce fluorinated products (rather than oxides). For example, mixtures of gaseous fluorine and methane are explosive, just like mixtures of oxygen and methane. Chlorine trifluoride is a strong fluorinating agent that ignites fuels more readily than oxygen.
[edit] Chemical equation
Generally, the chemical equation for stoichiometric burning of hydrocarbon in oxygen is as follows:
For example, the burning of propane is:
The simple word equation for the combustion of a hydrocarbon in oxygen is:
If the combustion takes place using air as the oxygen source, the corresponding equations are:
For example, the burning of propane is:
The simple word equation for the combustion of a hydrocarbon in air is:
[edit] Fuels
[edit] Liquid fuels
Combustion of a liquid fuel in an oxidizing atmosphere actually happens in the gas phase. It is the vapour that burns, not the liquid. Therefore, a liquid will normally catch fire only above a certain temperature, its flash point. The flash point of a liquid fuel is the lowest temperature at which it can form an ignitable mix with air. It is also the minimum temperature at which there is enough evaporated fuel in the air to start combustion.
[edit] Solid fuels
The act of combustion consists of three relatively distinct but overlapping phases:
- Preheating phase, when the unburned fuel is heated up to its flash point and then fire point. Flammable gases start being evolved in a process similar to dry distillation.
- Distillation phase or gaseous phase, when the mix of evolved flammable gases with oxygen is ignited. Energy is produced in the form of heat and light. Flames are often visible. Heat transfer from the combustion to the solid maintains the evolution of flammable vapours.
- Charcoal phase or solid phase, when the output of flammable gases from the material is too low for persistent presence of flame and the charred fuel does not burn rapidly anymore but just glows and later only smoulders.
[edit] Temperature
Assuming perfect combustion conditions, such as complete combustion under adiabatic conditions (i.e., no heat loss or gain), the adiabatic combustion temperature can be determined. The formula that yields this temperature is based on the first law of thermodynamics and takes note of the fact that the heat of combustion is used entirely for heating the fuel, the combustion air or oxygen, and the combustion product gases (commonly referred to as the flue gas).
In the case of fossil fuels burnt in air, the combustion temperature depends on
- the heating value
- the stoichiometric air to fuel ratio λ
- the heat capacity of fuel and air
- the air and fuel inlet temperatures
The adiabatic combustion temperature (also known as the adiabatic flame temperature) increases for higher heating values and inlet air and fuel temperatures and for stoichiometric air ratios approaching one.
Most commonly, the adiabatic combustion temperatures for coals are around 2200 °C (for inlet air and fuel at ambient temperatures and for λ = 1.0), around 2150 °C for oil and 2000 °C for natural gas.
In industrial fired heaters, power plant steam generators, and large gas-fired turbines, the more common way of expressing the usage of more than the stoichiometric combustion air is percent excess combustion air. For example, excess combustion air of 15 percent means that 15 percent more than the required stoichiometric air is being used.
[edit] Analysis
This section provides a combustion analysis for a few typical fuel cases (carbon, hydrogen, sulfur, coal, oil and gas) when the fuel reacts with air at stoichiometric conditions.
In the presented combustion analysis, both fuel and air are at inlet combustion conditions of 298 K and 1 atm of absolute pressure. Furthermore, combustion is complete and with no heat loss.
During the combustion, a large amount of reactants' chemical energy gets released in the form of thermal energy.
Enthalpy of combustion (HHV or higher heating value) is the difference between the reactants enthalpy value minus the combustion products enthalpy value at the reference temperature, which is 298 K.
When the reactants enthalpy value is equal to the combustion products enthalpy value, one can calculate the combustion products adiabatic flame temperature.
The plot in Figure 1 depicts the reactants and combustion products enthalpy value change with an increase in the temperature.
Physical properties for both reactants and combustion products are very important and need to be known in order to carry out successful combustion calculations.
The plot in Figure 2 depicts how the reactants and combustion products species enthalpy values change with the temperature. The physical properties provided in this plot come from the JANAF Thermochemical Data - Tables, 1970.
It is interesting to note that the enthalpy value for basic combustion elements such as carbon (C), hydrogen (H), sulfur (S), oxygen (O) and nitrogen (N) is equal to zero at the combustion conditions of 298 K and 1 atm.
Also, it should be mentioned that for ideal gas species, the enthalpy value is only dependent on the temperature.
In addition to knowing the reactants and combustion products physical properties, for any kind of combustion analysis and calculations, it is important to know both fuel and oxidant compositions.
For solid and liquid type fuels, the fuel compositions is given on the weight basis for a unit mass amount. In this analysis, CH4 is the only gas fuel considered. In order to keep the combustion analysis simple and straightforward, the CH4 composition is provided on the weight basis. Oxidant composition is usually given on the mole/volume basis.
Table 1 provides some fuel compositions:
| Fuel | C | H | S | N | O | H2O | CH4 |
|---|---|---|---|---|---|---|---|
| Carbon | 1.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | - |
| Hydrogen | 0.000 | 1.000 | 0.000 | 0.000 | 0.000 | 0.000 | - |
| Sulfur | 0.000 | 0.000 | 1.000 | 0.000 | 0.000 | 0.000 | - |
| Coal | 0.780 | 0.050 | 0.030 | 0.040 | 0.080 | 0.020 | - |
| Oil | 0.860 | 0.140 | 0.000 | 0.000 | 0.000 | 0.000 | - |
| Fuel Gas | - | - | - | - | - | - | 1.000 |
Table 2 provides the composition of air:
| Oxidant | N kg/kg | O kg/kg | N2 mol/mol | O2 mol/mol |
|---|---|---|---|---|
| Air | 0.767 | 0.233 | 0.790 | 0.210 |
Again, in this combustion analysis, only the stoichiometric combustion is analyzed. Results of such analysis are provided, including the combustion gas products composition on weight and mole/volume basis, the adiabatic flame temperature, the stoichiometric ratio and the fuel's higher heating value (HHV).
Table 3 provides the combustion gas products composition on a weight basis:
| Fuel | CO2 | H2O | SO2 | N2 | O2 |
|---|---|---|---|---|---|
| Carbon | 0.295 | 0.000 | 0.000 | 0.705 | 0.000 |
| Hydrogen | 0.000 | 0.255 | 0.000 | 0.745 | 0.000 |
| Sulfur | 0.000 | 0.000 | 0.378 | 0.622 | 0.000 |
| Coal | 0.249 | 0.041 | 0.005 | 0.705 | 0.000 |
| Oil | 0.203 | 0.079 | 0.000 | 0.718 | 0.000 |
| Fuel Gas | 0.151 | 0.124 | 0.000 | 0.725 | 0.000 |
Table 4 provides the combustion gas products composition on a volume or mole basis:
| Fuel | CO2 | H2O | SO2 | N2 | O2 |
|---|---|---|---|---|---|
| Carbon | 0.210 | 0.000 | 0.000 | 0.790 | 0.000 |
| Hydrogen | 0.000 | 0.347 | 0.000 | 0.653 | 0.000 |
| Sulfur | 0.000 | 0.000 | 0.210 | 0.789 | 0.000 |
| Coal | 0.170 | 0.068 | 0.002 | 0.759 | 0.000 |
| Oil | 0.133 | 0.127 | 0.000 | 0.740 | 0.000 |
| Fuel Gas | 0.095 | 0.190 | 0.000 | 0.715 | 0.000 |
When considering coal, oil and gas as the fuel, coal has the largest amount of CO2 in the combustion gas products on both weight and mole basis.
Table 5 provides the combustion adiabatic flame temperature, stoichiometric ratio and the fuel's higher heating value:
| Fuel | Adiabatic Flame Temperature (K) | Stoichiometric Ratio (see note below) | HHV (kJ/kg) |
|---|---|---|---|
| Carbon | 2,460 | 11.444 | 32,779.8 |
| Hydrogen | 2,525 | 34.333 | 141,866.8 |
| Sulfur (solid) | 1,972 | 4.292 | 9,261.3 |
| Coal | 2,484 | 10.487 | 32,937.9 |
| Oil | 2,484 | 14.580 | 47,630.0 |
| Fuel Gas | 2,327 | 17.167 | 50,151.2 |
| Note: Stoichiometric ratio is the mass of air required for complete combustion of a unit mass of fuel. Thus, 1 kg of carbon fuel requires 11.444 kg of air for complete, ideal combustion. | |||
Today, global warming is becoming more evident and it is being said that it is primarily caused by CO2 emissions. A detailed combustion analysis, as it is provided here, can be very useful in determining different fuel and technology scenarios that would result in the reduction of current CO2 emissions.
[edit] Instabilities
Combustion instabilities are typically violent pressure oscillations in a combustion chamber. These pressure oscillations can be as high as 180dB, and long term exposure to these cyclic pressure and thermal loads reduces the life of engine components. In rockets, such as the F1 used in the Saturn V program, instabilities led to massive damage of the combustion chamber and surrounding components. This problem was solved by re-designing the fuel injector. In liquid jet engines the droplet size and distribution can be used to attenuate the instabilities. Combustion instabilities are a major concern in ground-based gas turbine engines because of NOx emissions. The tendency is to run lean, an equivalence ratio less than 1, to reduce the combustion temperature and thus reduce the NOx emissions; however, running the combustor lean makes it very susceptible to combustion instabilities.
The Rayleigh Criterion is the basis for analysis of thermoacoustic combustion instabilities and is evaluated using the Rayleigh Index over one cycle of instability.
When the heat release oscillations are in phase with the pressure oscillations the Rayleigh Index is positive and the magnitude of the thermoacoustic instability increases. Consecutively if the Rayleigh Index is negative then thermoacoustic damping occurs. The Rayleigh Criterion implies that a thermoacoustic instability can be optimally controlled by having heat release oscillations 180 degrees out of phase with pressure oscillations at the same frequency. This minimizes the Rayleigh Index.