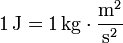Joule
From Wikipedia, the free encyclopedia
The joule (IPA: [dʒuːl] or [dʒaʊl]) (symbol: J) is the SI unit of energy. It was named after James Prescott Joule for his work on the relationship between heat, electricity and mechanical work.
Contents
|
[edit] Description
One joule is the work done, or energy expended, by a force of one newton moving an object one metre along the direction of the force. This quantity is also denoted as a Newton-meter with the symbol N·m. Note that torque also has the same units as work, but the quantities are not identical. In elementary units:
One joule is also:
- The work required to move an electric charge of one coulomb through an electrical potential difference of one volt; or one coulomb volt, with the symbol C·V.
- The work done to produce power of one watt continuously for one second; or one watt second (compare kilowatt-hour), with the symbol W·s.
[edit] History
A joule is the mechanical equivalent of heat meaning the number of units of work in which the unit of heat can perform. Its value was found by James Prescott Joule in experiments that showed the mechanical energy Joule's equivalent, and represented by the symbol J. The term was first introduced by Dr. Mayer of Heilbronn.
[edit] Conversions
1 joule is exactly 107 ergs.
1 joule is approximately equal to:
- 6.24150636309 ×1018 eV (electronvolts)
- 0.238845896628 cal (calorie) (small calories, lower case c)
- 2.390 ×10−4 kilocalorie, Calories (food energy, upper case C)
- 9.47817120313 ×10−4 BTU (British thermal unit)
- 0.737562149277 ft·lbf (foot-pound force)
- 23.7 ft·pdl (foot poundals)
- 2.7778 ×10−7 kilowatt-hour
- 2.7778 ×10−4 watt-hour
- 9.8692 ×10−3 litre-atmosphere
Units defined in terms of the joule include:
- 1 thermochemical calorie = 4.184 J
- 1 International Table calorie = 4.1868 J
- 1 watt-hour = 3600 J
- 1 kilowatt-hour = 3.6 ×106 J
Useful to remember:
- 1 joule = 1 newton-meter = 1 watt-second
1 joule in everyday life is approximately:
- the energy required to lift a small apple (102 g) one meter against Earth's gravity.
- the amount of energy, as heat, that a quiet person produces every hundredth of a second.
- the energy required to heat one gram of dry, cool air by 1 degree Celsius.
- one hundredth of the energy a person can get by drinking a single 5 mm diameter droplet of beer.
[edit] SI multiples
| Multiple | Name | Symbol | Multiple | Name | Symbol | |
|---|---|---|---|---|---|---|
| 100 | joule | J | ||||
| 101 | decajoule | daJ | 10–1 | decijoule | dJ | |
| 102 | hectojoule | hJ | 10–2 | centijoule | cJ | |
| 103 | kilojoule | kJ | 10–3 | millijoule | mJ | |
| 106 | megajoule | MJ | 10–6 | microjoule | µJ | |
| 109 | gigajoule | GJ | 10–9 | nanojoule | nJ | |
| 1012 | terajoule | TJ | 10–12 | picojoule | pJ | |
| 1015 | petajoule | PJ | 10–15 | femtojoule | fJ | |
| 1018 | exajoule | EJ | 10–18 | attojoule | aJ | |
| 1021 | zettajoule | ZJ | 10–21 | zeptojoule | zJ | |
| 1024 | yottajoule | YJ | 10–24 | yoctojoule | yJ |
| This SI unit is named after James Prescott Joule. As for all SI units whose names are derived from the proper name of a person, the first letter of its symbol is uppercase (J). But when an SI unit is spelled out, it should always be written in lowercase (joule), unless it begins a sentence or is the name "degree Celsius". — Based on The International System of Units, section 5.2. |