Mass number
From Wikipedia, the free encyclopedia
The mass number (A), also called atomic mass number (not to be confused with atomic number (Z) which denotes the number of protons in a nucleus) or nucleon number, is the number of nucleons (protons and neutrons) in an atomic nucleus. The mass number is unique for each isotope of an element and is written either after the element name or as a superscript to the left of an element's symbol. For example, carbon-12 (12C) has 6 protons and 6 neutrons. The full isotope symbol would also have the atomic number (Z) as a subscript to the left of the element symbol directly below the mass number: 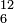 . Note that this is redundant, as there is a one-to-one mapping between atomic number and element symbol, so it is rarely used, except when we want to clarify the number of protons in a nucleus, such as in atomic reactions.
. Note that this is redundant, as there is a one-to-one mapping between atomic number and element symbol, so it is rarely used, except when we want to clarify the number of protons in a nucleus, such as in atomic reactions.
The difference between the mass number and the atomic number gives the number of neutrons (N) in a given nucleus: N=A−Z.
For example: Carbon-14 is created from Nitrogen-14 with seven protons (p) and seven neutrons via a cosmic ray interaction which transmutes 1 proton into 1 neutron. Thus the atomic number decreases by 1 (Z: 7→6) and the mass number remains the same (A = 14), however the number of neutrons increases by 1 (n: 7→8).
- Before: Nitrogen-14 (7p, 7n)
- After: Carbon-14 (6p, 8n).
This should not be confused with the relative atomic mass which is the average abundance atomic mass number of the differing isotopes found. For instance, there are two isotopes of chlorine: Chlorine-35 and Chlorine-37. In any given sample of chlorine that has not had any mass separation there will be roughly 75% of chlorine atoms which are chlorine-35 and only 25% of chlorine atoms which are Chlorine-37. This gives chlorine a relative atomic mass of 35.5 (actually 35.4527 g/mol).
Categories: Nuclear chemistry | Chemical properties
