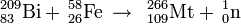Meitnerium
From Wikipedia, the free encyclopedia
| |||||||||||||
| General | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Name, Symbol, Number | meitnerium, Mt, 109 | ||||||||||||
| Chemical series | transition metals | ||||||||||||
| Group, Period, Block | 9, 7, d | ||||||||||||
| Appearance | unknown, probably silvery white or metallic gray | ||||||||||||
| Standard atomic weight | (278) g·mol−1 | ||||||||||||
| Electron configuration | perhaps [Rn] 5f14 6d7 7s2 (guess based on iridium) | ||||||||||||
| Electrons per shell | 2, 8, 18, 32, 32, 15, 2 | ||||||||||||
| Phase | presumably a solid | ||||||||||||
| CAS registry number | 54038-01-6 | ||||||||||||
| Selected isotopes | |||||||||||||
| |||||||||||||
| References | |||||||||||||
Meitnerium (IPA: /ˌmʌɪtˈnɛːriəm/), also called eka-iridium, is a chemical element in the periodic table that has the symbol Mt and atomic number 109. It is a synthetic element whose most stable isotope is Mt-276 with a half-life of 720 milliseconds.
[edit] History
Meitnerium was first synthesized on August 29, 1982 by a German research team led by Peter Armbruster and Gottfried Münzenberg at the Institute for Heavy Ion Research (Gesellschaft für Schwerionenforschung) in Darmstadt.
The team bombarded a target of bismuth-209 with accelerated nuclei of iron-58 and isotope meitnerium-266 was produced:
The synthesis of this element demonstrated that nuclear fusion techniques could be used to make new, heavy nuclei.
The name meitnerium was suggested in honor of the Austrian physicist and mathematician Lise Meitner, but there was an element naming controversy as to what the elements from 101 to 109 were to be called; thus IUPAC adopted unnilennium (IPA: /ˌjuːnɪˈlɛniəm/, symbol Une) as a temporary, systematic element name. In 1997, however, the dispute was resolved and the current name was adopted.