Henry's law
From Wikipedia, the free encyclopedia
In chemistry, Henry's law is one of the gas laws, formulated by William Henry. It states that:
- At a constant temperature, the amount of a given gas dissolved in a given type and volume of liquid is directly proportional to the partial pressure of that gas in equilibrium with that liquid.
Contents
|
[edit] Formula and Henry constant
A formula for Henry's Law is:
where:
 the partial pressure of the solute above the solution
the partial pressure of the solute above the solution the concentration of the solute in the solution (in one of its many units)
the concentration of the solute in the solution (in one of its many units) the Henry's Law constant, which has units such as L·atm/mol, atm/(mol fraction) or Pa·m3/mol.
the Henry's Law constant, which has units such as L·atm/mol, atm/(mol fraction) or Pa·m3/mol.
Taking the natural logarithm of the formula, gives us the more commonly used formula:[1]
Some values for k include:
- oxygen (O2) : 769.2 L·atm/mol
- carbon dioxide (CO2) : 29.4 L·atm/mol
- hydrogen (H2) : 1282.1 L·atm/mol
when these gases are dissolved in water at 298 kelvin.
Note that in the above, the unit of concentration was chosen to be molarity. Hence the dimensional units: L is liters of solution, atm is the partial pressure of the gaseous solute above the solution (in atmospheres of absolute pressure), and mol is the moles of the gaseous solute in the solution. Also note that the Henry's Law constant, k, varies with the solvent and the temperature.
As discussed in the next section, there are other forms of Henry's Law each of which defines the constant k differently and requires different dimensional units. The form of the equation presented above is consistent with the given example numerical values for oxygen, carbon dioxide and hydrogen and with their corresponding dimensional units.
[edit] Other Forms of Henry's Law
There are various other forms Henry's Law which are discussed in the technical literature.[2][3]
| equation: |  |  |  |  |
|---|---|---|---|---|
| dimension: | ![\left[\frac{\mathrm{L}_{soln} \cdot \mathrm{atm}}{\mathrm{mol}_{gas}}\right]](http://upload.wikimedia.org/math/4/a/4/4a445487a0b99e223435f4f615439be1.png) | ![\left[\frac{\mathrm{mol}_{gas}}{\mathrm{L}_{soln} \cdot \mathrm{atm}}\right]](http://upload.wikimedia.org/math/5/3/5/53568bd4a739e91881d7db6ad3db3f2a.png) | ![\left[\frac{\mathrm{atm} \cdot (\mathrm{mol}_{water}+ \mathrm{mol}_{gas})}{\mathrm{mol}_{gas}}\right]](http://upload.wikimedia.org/math/d/9/e/d9e459364f99806afc4634604bd34346.png) | ![\left[ \text{dimensionless} \right]](http://upload.wikimedia.org/math/0/f/f/0ff7ff84b5ec0627f0b384ded7b54498.png) |
| O2 | 769.23 | 1.3 E-3 | 4.259 E4 | 3.180 E-2 |
| H2 | 1282.05 | 7.8 E-4 | 7.099 E4 | 1.907 E-2 |
| CO2 | 29.41 | 3.4 E-2 | 0.163 E4 | 0.8317 |
| N2 | 1639.34 | 6.1 E-4 | 9.077 E4 | 1.492 E-2 |
| He | 2702.7 | 3.7 E-4 | 14.97 E4 | 9.051 E-3 |
| Ne | 2222.22 | 4.5 E-4 | 12.30 E4 | 1.101 E-2 |
| Ar | 714.28 | 1.4 E-3 | 3.955 E4 | 3.425 E-2 |
| CO | 1052.63 | 9.5 E-4 | 5.828 E4 | 2.324 E-2 |
where:
 = moles of gas per liter of solution
= moles of gas per liter of solution = liters of solution
= liters of solution = partial pressure of gas above the solution, in atmospheres of absolute pressure
= partial pressure of gas above the solution, in atmospheres of absolute pressure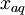 = mole fraction of gas in solution = moles of gas per total moles ≈ moles of gas per mole of water
= mole fraction of gas in solution = moles of gas per total moles ≈ moles of gas per mole of water = atmospheres of absolute pressure
= atmospheres of absolute pressure
As can be seen by comparing the equations in the above table, the Henry's Law constant kH,pc is simply the inverse of the constant kH,cp. Since all kH may be referred to as the Henry's Law constant, readers of the technical literature must be quite careful to note which version of the Henry's Law equation is being used.
It should also be noted the Henry's Law is a limiting law that only applies for dilute enough solutions. The range of concentrations in which it applies becomes narrower the more the system diverges from non-ideal behavior. Roughly speaking, that is the more chemically different the solute is from the solvent.
It also only applies for solutions where the solvent does not react chemically with the gas being dissolved. A common example of a gas that does react with the solvent is carbon dioxide, which rapidly forms hydrated carbon dioxide and then carbonic acid (H2CO3) with water.
[edit] Temperature dependence of the Henry constant
When the temperature of a system changes, the Henry constant will also change. This is why some people prefer to name it Henry coefficient. There are multiple equations assessing the effect of temperature on the constant. A simple example is [3], which is a form of the van 't Hoff equation:
where
- k for a given temperature is the Henry's Law constant (as defined in the first section of this article), identical with kH,pc defined in Table 1,
- T is in kelvins,
- the index Θ (Theta) refers to the standard temperature (298K).
The above equation is an approximation only and should be used only when no better experimentally derived formula for a given gas exists.
The following table lists some values for constant C (dimension of kelvins) in the equation above:
| Gas | O2 | H2 | CO2 | N2 | He | Ne | Ar | CO |
| C | 1700 | 500 | 2400 | 1300 | 230 | 490 | 1300 | 1300 |
Because solubility of gases is decreasing with increasing temperature, the partial pressure a given gas concentration has in liquid must increase. While heating water (saturated with nitrogen) from 25°C to 95°C the solubility will decrease to about 43% of its initial value. This can be verified when heating water in a pot. Small bubbles evolve and rise, long before the water reaches boiling temperature. Similarly, carbon dioxide from a carbonated drink escapes much faster when the drink is not cooled because of the increased partial pressure of CO2 in higher temperatures. Partial pressure of CO2 in seawater doubles with every 16 K increase in temperature.[4]
The constant C may be regarded as:
where
 is the enthalpy of solution
is the enthalpy of solution- R is the gas constant.
[edit] Henry's law in geophysics
In geophysics a version of Henry's law applies to the solubility of a noble gas in contact with silicate melt. One equation used is
where:
- subscript m = melt
- subscript g = gas phase
- ρ = the number densities of the solute gas in the melt and gas phase
- β = 1 / kBT an inverse temperature scale
- kB = the Boltzmann constant
- μex,m and μex,g = the excess chemical potential of the solute in the two phases.
[edit] Henry's law versus Raoult's law
Both Henry's law and Raoult's law state that the vapor pressure of a component, p, is proportional to its concentration.
- Henry's law:
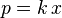
- Raoult's law:

where:
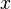 is the mole fraction of the component;
is the mole fraction of the component;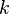 is the Henry constant; (Note that the numerical value and dimensions of this constant change when mole fractions are used rather than molarity, as seen in Table 1.)
is the Henry constant; (Note that the numerical value and dimensions of this constant change when mole fractions are used rather than molarity, as seen in Table 1.)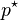 is the equilibrium vapor pressure of the pure component.
is the equilibrium vapor pressure of the pure component.
If the solution is ideal, both components follow Raoult's law over the entire composition range, but Henry noticed that at low concentrations of non-ideal solutions, the constant of proportionality is not p*. Therefore Henry's law uses an empirically-derived constant, k, based on an infinitely-dilute solution, i.e. x = 0, that is specific to the components in the mixture and the temperature.
In most systems, the laws can only be applied over very limited concentrations at the extreme ends of the mole-fraction range. Raoult's law, which uses the vapor pressure of the pure component, is best used for the major component (solvent) and in mixtures of similar components. Henry's law applies to the minor component (solute) in dilute solutions.
In ideal-dilute solutions, the minor component follows Henry's law, while the solvent obeys Raoult's law. This is proved by the Gibbs-Duhem equation.


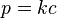
![k(T) = k(T_\Theta) \cdot e^{ \left[ -C \cdot \left( \frac{1}{T}-\frac{1}{T_\Theta}\right)\right]}\,](http://upload.wikimedia.org/math/4/b/a/4bac643dfaa134b6b82ab822cbd38b44.png)

