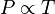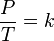Gay-Lussac's law
From Wikipedia, the free encyclopedia
Gay-Lussac's law is one of two laws named after the French chemist Joseph Louis Gay-Lussac, which relate to the properties of gases and are known by the same name.
[edit] Law of combining volumes
Gay-Lussac's law, known as the law of combining volumes, states that:
- The ratio between the combining volumes of gases and the product, if gaseous, can be expressed in small whole numbers
Gay-Lussac discovered this law in 1809. This played a major role in the development of modern gas stoichiometry because in 1811, Avogadro used Gay-Lussac’s Law to form Avogadro's hypothesis.
[edit] Other law
The other law, discovered in 1802, states that:
- The pressure of a fixed amount of gas at fixed volume is directly proportional to its temperature in kelvins.
It is expressed mathematically as:
or
where:
- P is the pressure of the gas.
- T is the temperature of the gas (measured in kelvins).
- k is a constant.
This law holds true because temperature is a measure of the average kinetic energy of a substance; as the kinetic energy of a gas increases, its particles collide with the container walls more rapidly, thereby exerting increased pressure.
Simply put, if you increase the temperature you increase the pressure.
For comparing the same substance under two different sets of conditions, the law can be written as:
Charles's Law was also known as the Law of Charles and Gay-Lussac, because Charles used some of Gay-Lussac's data to formulate his law. However, in recent years the term has fallen out of favor since Gay-Lussac has the second but related law presented here attributed to him. This related form of Gay-Lussac's Law, Charles's Law, and Boyle's law form the combined gas law. The three gas laws in combination with Avogadro's Law can be generalized by the ideal gas law.