Pauli exclusion principle
From Wikipedia, the free encyclopedia
| Quantum physics |
 |
| Quantum mechanics |
| Introduction to... |
| Fundamental concepts |
| Decoherence · Interference |
| Experiments |
| Double-slit experiment |
| Equations |
| Schrödinger equation |
| Advanced theories |
| Quantum field theory |
| Interpretations |
| Copenhagen · Ensemble |
| Scientists |
| Planck · Schrödinger |
| |
The Pauli exclusion principle is a quantum mechanical principle formulated by Wolfgang Pauli in 1925. This principle is significant, because it explains why matter occupies space exclusively for itself and does not allow other material objects to pass through it, while at the same time allowing light and radiation to pass. It states that no two identical fermions may occupy the same quantum state simultaneously. A more rigorous statement of this principle is that, for two identical fermions, the total wave function is anti-symmetric. For electrons in a single atom, it states that no two electrons can have the same four quantum numbers, that is, if n, l, and ml are the same, ms must be different such that the electrons have opposite spins.
The Pauli exclusion principle mathematically follows from applying the rotation operator to two identical particles with half-integer spin.
Contents
|
[edit] Overview
The Pauli exclusion principle is one of the most important principles in physics, primarily because the three types of particles from which ordinary matter is made—electrons, protons, and neutrons—are all subject to it; consequently, all material particles exhibit space-occupying behavior. The Pauli exclusion principle underpins many of the characteristic properties of matter from the large-scale stability of matter to the existence of the periodic table of the elements.
The Pauli exclusion principle follows mathematically from the definition of the angular momentum operator (rotation operator) in quantum mechanics. The exchange of particles in the system of two identical particles (which is mathematically equivalent to the rotation of each particle by 180 degrees) results either in the change of the sign of wave function of the system (when the particles have half-integer spin) or not (when the particles have integer spin). Thus, no two identical particles of half integer spin can be at the same quantum place - because the wave function of such system must be equal to its opposite - and the only wave function which satisfies this condition is the zero wave function.
Particles with antisymmetric wave functions are called fermions—and obey the Pauli exclusion principle. Apart from the familiar electron, proton and neutron, these include neutrinos and quarks (from which protons and neutrons are made), as well as some atoms like helium-3. All fermions possess "half-integer spin", meaning that they possess an intrinsic angular momentum whose value is 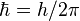 (Planck's constant divided by 2π) times a half-integer (1/2, 3/2, 5/2, etc.). In the theory of quantum mechanics, fermions are described by "antisymmetric states", which are explained in greater detail in the article on identical particles.
(Planck's constant divided by 2π) times a half-integer (1/2, 3/2, 5/2, etc.). In the theory of quantum mechanics, fermions are described by "antisymmetric states", which are explained in greater detail in the article on identical particles.
Particles with integer spin have a symmetric wave function and are called bosons; in contrast to fermions, they may share the same quantum states. Examples of bosons include the photon and the W and Z bosons.
[edit] History
In the early 20th century, it became evident that pairs of electrons or even numbers of electrons are more stable than odd numbers. In the famous 1916 article The Atom and the Molecule by Gilbert N. Lewis, for example, rule three of his six postulates of chemical behavior states that the atom tends to hold an even number of electrons in the shell and especially to hold eight electrons which are normally arranged symmetrically at the eight corners of a cube (see: cubical atom).
Building on these and other views, the Pauli exclusion principle was originally formulated as an empirical principle. It was invented by Pauli in 1924 to explain experimental results in the Zeeman effect in atomic spectroscopy, ferromagnetism, and how the periodic table is regulated by the electron structure of atoms, well before the 1925 formulation of the modern theory of quantum mechanics by Werner Heisenberg and Erwin Schrödinger. However, this does not mean that the principle is in any way approximate or unreliable; in fact, it is one of the most well-tested and commonly-accepted results in physics.
[edit] Connection to quantum state symmetry
The Pauli exclusion principle can be derived starting from the assumption that a system of particles occupy antisymmetric quantum states. According to the spin-statistics theorem, particles with integer spin occupy symmetric quantum states, and particles with half-integer spin occupy antisymmetric states; furthermore, only integer or half-integer values of spin are allowed by the principles of quantum mechanics.
As discussed in the article on identical particles, an antisymmetric two-particle state in which one particle exists in state  (nota) and the other in state
(nota) and the other in state  is
is
However, if  and
and  are just the same state, the above formula gives the zero set:
are just the same state, the above formula gives the zero set:
This does not represent a valid quantum state, because the state vectors representing quantum states must be normalizable to 1. In other words, we can never find the particles in this system occupying the same quantum state.
[edit] Consequences
The Pauli exclusion principle helps explain a wide variety of physical phenomena. One such phenomenon is the "rigidity" or "stiffness" of ordinary matter (fermions): the principle states that identical fermions cannot be squeezed into each other (cf. Young and bulk moduli of solids), hence our everyday observations in the macroscopic world that material objects collide rather than passing straight through each other, and that we are able to stand on the ground without sinking through it. Another consequence of the principle is the elaborate electron shell structure of atoms and of the way atoms share electron(s) - thus variety of chemical elements and of their combinations (chemistry). (An electrically neutral atom contains bound electrons equal in number to the protons in the nucleus. Since electrons are fermions, the Pauli exclusion principle forbids them from occupying the same quantum state, so electrons have to "pile on top of each other" within an atom).
For example, consider a neutral helium atom, which has two bound electrons. Both of these electrons can occupy the lowest-energy (1s) states by acquiring opposite spin. This does not violate the Pauli principle because spin is part of the quantum state of the electron, so the two electrons are occupying different quantum states. However, the spin can take only two different values (or eigenvalues). In a lithium atom, which contains three bound electrons, the third electron cannot fit into a 1s state, and has to occupy one of the higher-energy 2s states instead. Similarly, successive elements produce successively higher-energy shells. The chemical properties of an element largely depend on the number of electrons in the outermost shell, which gives rise to the periodic table of the elements.
In conductors and semi-conductors free electrons have to share entire bulk space - thus their energy levels are stacking up creating band structure out of each atomic energy level. In strong conductors (metals) electrons are so degenerate that they can not even contribute much into thermal capacity of a metal. Many mechanical, electrical, magnetic, optical and chemical properties of solids are the direct consequence of Pauli repulsion of free and semi-free electrons.
Astronomy provides another spectacular demonstration of this effect, in the form of white dwarf stars and neutron stars. For both such bodies, their usual atomic structure is disrupted by large gravitational forces, leaving the constituents supported by "degeneracy pressure" alone. This exotic form of matter is known as degenerate matter. In white dwarfs, the atoms are held apart by the degeneracy pressure of the electrons. In neutron stars, which exhibit even larger gravitational forces, the electrons have merged with the protons to form neutrons, which produce a larger degeneracy pressure. Neutrons are the most "rigid" objects known - their Young modulus (or more accurately, bulk modulus) is 20 orders of magnitude larger than that of diamond.
According to general relativity, in the centers of black holes the gravitational forces would become so intense that everything would break down into fundamental particles, which are supposedly point-like with no internal structure. All of these particles could then pile up at one zero-dimensional point because the gravitational forces would be greater than the degeneracy pressure . This would seem to violate the Pauli exclusion principle, but since the interiors of black holes are beyond the event horizon, and thus inaccessible to experimental verification, this hypothesis remains untested. [citation needed]


