Quantum number
From Wikipedia, the free encyclopedia
Quantum numbers describe values of conserved numbers in the dynamics of the quantum system. They often describe specifically the energies of electrons in atoms, but other possibilities include angular momentum, spin etc. Since any quantum system can have one or more quantum numbers, it is a futile job to list all possible quantum numbers.
Contents
|
[edit] How many quantum numbers?
The question of how many quantum numbers are needed to describe any given system has no universal answer, although for each system one must find the answer for a full analysis of the system. The dynamics of any quantum system are described by a quantum Hamiltonian, H. There is one quantum number of the system corresponding to the energy, i.e., the eigenvalue of the Hamiltonian. There is also one quantum number for each operator O that commutes with the Hamiltonian (i.e. satisfies the relation OH = HO). These are all the quantum numbers that the system can have. Note that the operators O defining the quantum numbers should be independent of each other. Often there is more than one way to choose a set of independent operators. Consequently, in different situations different sets of quantum numbers may be used for the description of the same system.
[edit] Single electron in an atom
- This section is not meant to be a full description of this problem. For that, see the article on the Hydrogen-like atom, Bohr atom, Schrödinger equation and the Dirac equation.
The most widely studied set of quantum numbers is that for a single electron in an atom: not only because it is useful in chemistry, being the basic notion behind the periodic table, Valence and a host of other properties, but also because it is a solvable and realistic problem, and, as such, finds widespread use in textbooks.
In non-relativistic quantum mechanics the Hamiltonian of this system consists of the kinetic energy of the electron and the potential energy due to the Coulomb force between the nucleus and the electron. The kinetic energy can be separated into a piece which is due to angular momentum, J, of the electron around the nucleus, and the remainder. Since the potential is spherically symmetric, the full Hamiltonian commutes with J2. J2 itself commutes with any one of the components of the angular momentum vector, conventionally taken to be Jz. These are the only mutually commuting operators in this problem; hence, there are three quantum numbers.
These are conventionally known as
- The principal quantum number (n = 1, 2, 3, 4 ...) denotes the eigenvalue of H with the J2 part removed. This number therefore has a dependence only on the distance between the electron and the nucleus (ie, the radial coordinate, r). The average distance increases with n, and hence quantum states with different principal quantum numbers are said to belong to different shells.
- The azimuthal quantum number (l = 0, 1 ... n−1) (also known as the angular quantum number or orbital quantum number) gives the orbital angular momentum through the relation
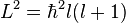 . In chemistry, this quantum number is very important, since it specifies the shape of an atomic orbital and strongly influences chemical bonds and bond angles. In some contexts, l=0 is called an s orbital, l=1, a p orbital, l=2, a d orbital and l=3, an f orbital.
. In chemistry, this quantum number is very important, since it specifies the shape of an atomic orbital and strongly influences chemical bonds and bond angles. In some contexts, l=0 is called an s orbital, l=1, a p orbital, l=2, a d orbital and l=3, an f orbital. - The magnetic quantum number (ml = −l, −l+1 ... 0 ... l−1, l) is the eigenvalue,
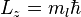 . This is the projection of the orbital angular momentum along a specified axis.
. This is the projection of the orbital angular momentum along a specified axis.
Results from spectroscopy indicated that up to two electrons can occupy a single orbital. However two electrons can never have the same exact quantum state and can never have the same set of quantum numbers. A fourth quantum number with two possible values was added as an ad hoc assumption to resolve the conflict. It could later be explained in detail by relativistic quantum mechanics.
- The spin quantum number (ms = −1/2 or +1/2), the intrinsic angular momentum of the electron. This is the projection of the spin s=1/2 along the specified axis.
To summarize, the quantum state of an electron is determined by the quantum numbers:
| name | symbol | orbital meaning | range of values | value example |
|---|---|---|---|---|
| principal quantum number |  | shell |  |  |
| azimuthal quantum number (angular momentum) |  | subshell |  | for  : : |
| magnetic quantum number, (projection of angular momentum) | 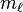 | energy shift |  | for 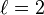 : : |
| spin quantum number |  | spin | 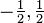 | always only: 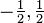 |
Example: The quantum numbers used to refer to the outermost valence electron of the Fluorine (F) atom, which is located in the 2p atomic orbital, are; n = 2, l = 1, ml = 1, or 0, or −1, ms = −1/2 or 1/2.
Note that molecular orbitals require totally different quantum numbers, because the Hamiltonian and its symmetries are quite different.
[edit] Quantum numbers with spin-orbit interaction
- For more details on this topic, see Clebsch-Gordan coefficients.
When one takes the spin-orbit interaction into consideration, l, m and s no longer commute with the Hamiltonian, and their value therefore changes over time. Thus another set of quantum numbers should be used. This set includes
- The total angular momentum quantum number (j = 1/2,3/2 ... n−1/2) gives the total angular momentum through the relation
 .
. - The projection of the total angular momentum along a specified axis (mj = -j,-j+1... j), which is analogous to m, and satisfies mj = ml + ms.
- Parity. This is the eigenvalue under reflection, and is positive (i.e. +1) for states which came from even l and negative (i.e. -1) for states which came from odd l. The former is also known as even parity and the latter as odd parity
For example, consider the following eight states, defined by their quantum numbers:
- (1) l = 1, ml = 1, ms = +1/2
- (2) l = 1, ml = 1, ms = -1/2
- (3) l = 1, ml = 0, ms = +1/2
- (4) l = 1, ml = 0, ms = -1/2
- (5) l = 1, ml = -1, ms = +1/2
- (6) l = 1, ml = -1, ms = -1/2
- (7) l = 0, ml = 0, ms = +1/2
- (8) l = 0, ml = 0, ms = -1/2
The quantum states in the system can be described as linear combination of these eight states. However, in the presence of spin-orbit interaction, if one wants to describe the same system by eight states which are eigenvectors of the Hamiltonian (i.e. each represents a state which does not mix with others over time), we should consider the following eight states:
- j = 3/2, mj = 3/2, even parity (coming from state (1) above)
- j = 3/2, mj = 1/2, even parity (coming from states (2) and (3) above)
- j = 3/2, mj = -1/2, even parity (coming from states (4) and (5) above)
- j = 3/2, mj = -3/2, even parity (coming from state (6))
- j = 1/2, mj = 1/2, even parity (coming from state (2) and (3) above)
- j = 1/2, mj = -1/2, even parity (coming from states (4) and (5) above)
- j = 1/2, mj = 1/2, odd parity (coming from state (7) above)
- j = 1/2, mj = -1/2, odd parity (coming from state (8) above)
[edit] Elementary particles
For a more complete description of the quantum states of elementary particles see the articles on the standard model and flavour (particle physics).
Elementary particles contain many quantum numbers which are usually said to be intrinsic to them. However, it should be understood that the elementary particles are quantum states of the standard model of particle physics, and hence the quantum numbers of these particles bear the same relation to the Hamiltonian of this model as the quantum numbers of the Bohr atom does to its Hamiltonian. In other words, each quantum number denotes a symmetry of the problem. It is more useful in field theory to distinguish between spacetime and internal symmetries.
Typical quantum numbers related to spacetime symmetries are spin (related to rotational symmetry), the parity, C-parity and T-parity (related to the Poincare symmetry of spacetime). Typical internal symmetries are lepton number and baryon number or the electric charge. For a full list of quantum numbers of this kind see the article on flavour.
It is worth mentioning here a minor but often confusing point. Most conserved quantum numbers are additive. Thus, in an elementary particle reaction, the sum of the quantum numbers should be the same before and after the reaction. However, some, usually called a parity, are multiplicative; ie, their product is conserved. All multiplicative quantum numbers belong to a symmetry (like parity) in which applying the symmetry transformation twice is equivalent to doing nothing. These are all examples of an abstract group called Z2.
[edit] References and external links
[edit] General principles
- Dirac, Paul A.M. (1982). Principles of quantum mechanics. Oxford University Press. ISBN 0-19-852011-5.
[edit] Atomic physics
- Quantum Numbers and Electron Configurations
- Quantum numbers for the hydrogen atom
- Lecture notes on quantum numbers
[edit] Particle physics
- Griffiths, David J. (2004). Introduction to Quantum Mechanics (2nd ed.). Prentice Hall. ISBN 0-13-805326-X.
- Halzen, Francis and Martin, Alan D. (1984). QUARKS AND LEPTONS: An Introductory Course in Modern Particle Physics. John Wiley & Sons. ISBN 0-471-88741-2.
- The particle data group
Categories: Quantum mechanics | Fundamental physics concepts
