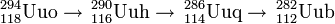Ununbium
From Wikipedia, the free encyclopedia
| |||||||||||||
| General | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Name, Symbol, Number | ununbium, Uub, 112 | ||||||||||||
| Chemical series | transition metals | ||||||||||||
| Group, Period, Block | 12, 7, d | ||||||||||||
| Appearance | unknown, probably silvery white or metallic gray liquid or colorless gas | ||||||||||||
| Standard atomic weight | (288) g·mol−1 | ||||||||||||
| Electron configuration | perhaps [Rn] 5f14 6d10 7s2 (guess based on mercury) | ||||||||||||
| Electrons per shell | 2, 8, 18, 32, 32, 18, 2 | ||||||||||||
| Phase | unknown; possibly gas | ||||||||||||
| CAS registry number | 54084-26-3 | ||||||||||||
| Selected isotopes | |||||||||||||
| |||||||||||||
| References | |||||||||||||
Ununbium (IPA: /ˌjuːˈnʌnbiəm/), or eka-mercury, is a temporary IUPAC systematic element name for a chemical element in the periodic table that has the temporary symbol Uub and the atomic number 112. Element 112 is one of the superheavy elements. Following periodic trends, one might expect a liquid metal more volatile than mercury. However, some experimental work so far indicates a gas[1] and theoretical considerations also point to properties more similar to a noble gas than to mercury.[1][2]
Ununbium was first created on February 9, 1996 at the Gesellschaft für Schwerionenforschung (GSI) in Darmstadt, Germany. This element was created by fusing a zinc-70 nucleus with a lead-208 nucleus by accelerating zinc nuclei into a lead target in a heavy ion accelerator. The two ununbium nuclei so produced had a mass number of 277 [3].
The element was synthesized in 2000 and 2004 in the Joint Institute for Nuclear Research, Russia.
In May 2006 in the JINR synthesis of this element was confirmed by another method. The isotope 282Uub was identified as a final product of this series of alpha decays:
It was found that the final nucleus undergoes spontaneous fission.[4] Spontaneous fission products are much smaller and irregular.