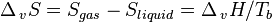Enthalpy of vaporization
From Wikipedia, the free encyclopedia
The enthalpy of vaporization, (symbol ΔvH), also known as the heat of vaporization or heat of evaporation, is the energy required to transform a given quantity of a substance into a gas. It is measured at the boiling point of the substance, although tabulated values are usually corrected to 298 K: the correction is small, and is often smaller than the uncertainty in the measured value. Values are usually quoted in kJ/mol, although kJ/kg, kcal/mol, cal/g and Btu/lb are also possible, among others.
The enthalpy of condensation (or heat of condensation) is numerically exactly equal to the enthalpy of vaporization, but has the opposite sign: enthalpy changes of vaporization are always positive (heat is absorbed by the substance), whereas enthalpy changes of condensation are always negative (heat is released by the substance).
The enthalpy of vaporization can be viewed as the energy required to overcome the intermolecular interactions in the liquid (or solid, in the case of sublimation). Hence helium has a particularly low enthalpy of vaporization, 0.0845 kJ/mol, as the van der Waals forces between helium atoms are particularly weak. On the other hand, the molecules in liquid water are held together by relatively strong hydrogen bonds, and its enthalpy of vaporization, 40.8 kJ/mol, is more than five times the energy required to heat the same quantity of water from 0 °C to 100 °C (cp = 75.3 J K−1 mol−1). Care must be taken, however, when using enthalpies of vaporization to measure the strength of intermolecular forces, as these forces may persist to an extent in the gas phase (as is the case with hydrogen fluoride), and so the calculated value of the bond strength will be too low. This is particularly true of metals, which often form covalently bonded molecules in the gas phase: in these cases, the enthalpy of atomization must be used to obtain a true value of the bond energy.
An alternative description is to view the enthalpy of condensation as the heat which must be released to the surroundings to compensate for the drop in entropy when a gas condenses to a liquid. As the liquid and gas are in equilibrium at the boiling point (Tb), ΔvG = 0, which leads to:
As neither entropy nor enthalpy vary greatly with temperature, it is normal to use the tabulated standard values without any correction for the difference in temperature from 298 K. A correction must be made if the pressure is different from 100 kPa, as the entropy of a gas is proportional to its pressure (or, more precisely, to its fugacity): the entropies of liquids vary little with pressure, as the compressibility of a liquid is small.
These two definitions are equivalent: the boiling point is the temperature at which the increased entropy of the gas phase overcomes the intermolecular forces. As a given quantity of matter always has a higher entropy in the gas phase than in a condensed phase ( is always positive), and from
is always positive), and from
 ,
,
the Gibbs free energy change falls with increasing temperature: gases are favored at higher temperatures, as is observed in practice.
Contents
|
[edit] Selected values
[edit] Elements
Enthalpies of vaporization of the elements in kJ/mol
| Group → | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ↓ Period | ||||||||||||||||||||
| 1 | H 0.44936 | He 0.0845 | ||||||||||||||||||
| 2 | Li 145.92 | Be 292.40 | B 489.7 | C 355.8 | N 2.7928 | O 3.4099 | F 3.2698 | Ne 1.7326 | ||||||||||||
| 3 | Na 96.96 | Mg 127.4 | Al 293.4 | Si 384.22 | P 12.129 | S 1.7175 | Cl 10.2 | Ar 6.447 | ||||||||||||
| 4 | K 79.87 | Ca 153.6 | Sc 314.2 | Ti 421 | V 452 | Cr 344.3 | Mn 226 | Fe 349.6 | Co 376.5 | Ni 370.4 | Cu 300.3 | Zn 115.3 | Ga 258.7 | Ge 330.9 | As 34.76 | Se 26.3 | Br 15.438 | Kr 9.029 | ||
| 5 | Rb 72.216 | Sr 144 | Y 363 | Zr 581.6 | Nb 696.6 | Mo 598 | Tc 660 | Ru 595 | Rh 493 | Pd 357 | Ag 250.58 | Cd 100 | In 231.5 | Sn 295.8 | Sb 77.14 | Te 52.55 | I 20.752 | Xe 12.636 | ||
| 6 | Cs 67.74 | Ba 142 | * | Hf 575 | Ta 743 | W 824 | Re 715 | Os 627.6 | Ir 604 | Pt 510 | Au 334.4 | Hg 59.229 | Tl 164.1 | Pb 177.7 | Bi 104.8 | Po 60.1 | At 114 | Rn 16.4 | ||
| 7 | Fr n/a | Ra 37 | ** | Rf n/a | Db n/a | Sg n/a | Bh n/a | Hs n/a | Mt n/a | Ds n/a | Rg n/a | Uub n/a | Uut n/a | Uuq n/a | Uup n/a | Uuh n/a | Uus n/a | Uuo n/a | ||
| * Lanthanides | La 414 | Ce 414 | Pr n/a | Nd n/a | Pm n/a | Sm n/a | Eu n/a | Gd n/a | Tb n/a | Dy n/a | Ho n/a | Er n/a | Tm n/a | Yb n/a | Lu n/a | |||||
| ** Actinides | Ac n/a | Th 514.4 | Pa n/a | U n/a | Np n/a | Pu n/a | Am n/a | Cm n/a | Bk n/a | Cf n/a | Es n/a | Fm n/a | Md n/a | No n/a | Lr n/a | |||||
| 0–10 kJ/mol | 10–100 kJ/mol | 100–300 kJ/mol | >300 kJ/mol |
[edit] Other common substances
Common substances sorted by heat of vaporization:
| Compound | Heat of vaporization (kJ/mol) |
|---|---|
| Water | 40.65 (540 calories per gram) |
| Ethanol | 38.6 |
| Methanol | 37.4 |
| Ammonia | 23.35 |
| Butane | 21.0 (362 kJ/kg) |
| Propane | 15.7 (356 kJ/kg) |
| Phosphine | 14.6 |
| Methane | 8.19 |
| Compound | Heat of vaporization (kJ/mol) |